Urea Cycle Disorder Treatment Market Size 2025-2029
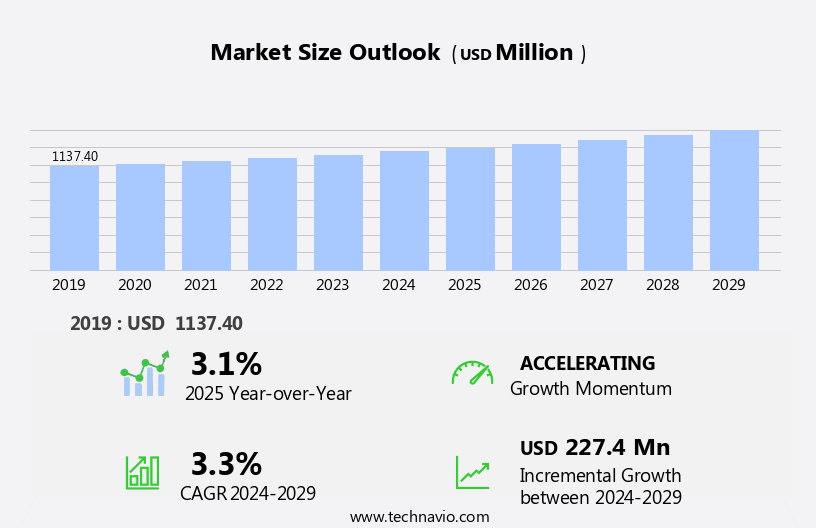
The urea cycle disorder treatment market size is forecast to increase by USD 227.4 million, at a CAGR of 3.3% between 2024 and 2029.
- The market is experiencing significant growth due to the increasing prevalence of urea cycle disorders. This condition, characterized by the inability to process ammonia in the body, affects approximately one in 25,000 people. The expanding patient population necessitates the development of effective treatment modalities, creating a promising growth avenue for market participants. Moreover, emerging economies present a substantial growth potential for the market. With improving healthcare infrastructure and services and rising awareness about these disorders, there is a growing demand for specialized care in these regions.
- However, low access to such care remains a substantial challenge. Addressing this issue through collaborations between governments, non-profit organizations, and healthcare providers could help improve patient outcomes and expand market opportunities. Companies seeking to capitalize on these trends should focus on developing affordable, accessible, and effective treatment solutions while navigating the challenges posed by limited healthcare infrastructure in emerging economies.
What will be the Size of the Urea Cycle Disorder Treatment Market during the forecast period?
Explore in-depth regional segment analysis with market size data - historical 2019-2023 and forecasts 2025-2029 - in the full report.
Request Free Sample
The urea cycle disorder (UCD) treatment market continues to evolve, driven by advancements in gene therapy, nitrogen scavenger therapy, and diagnostic tools. Neurological impacts of UCDs, such as developmental delay and cognitive developmental issues, necessitate early intervention and ongoing management. Genetic testing plays a crucial role in identifying UCDs, including argininosuccinate synthetase deficiency and ornithine transcarbamylase deficiency. The market for UCD treatments is expected to grow significantly, with industry analysts projecting a robust expansion in the coming years. For instance, the successful implementation of enzyme replacement therapy for phenylketonuria (PKU), a type of UCD, has led to improved patient survival and better disease management.
The Urea Cycle Defects (UCD) market is evolving with growing focus on early detection, therapeutic innovation, and comprehensive care ucd. Diagnosis often involves plasma amino acid analysis, blood ammonia measurement, enzyme activity assay, and molecular diagnosis ucd to confirm metabolic disruptions. Clinically, neurological symptoms ucd are common and linked to elevated ammonia levels. Treatment includes both pharmacological approach ucd and non-pharmacological approach ucd, supported by nutritional management ucd, emergency treatment ucd, and managing drug interactions ucd. A multidisciplinary team ucd and interdisciplinary care ucd are crucial for managing complications ucd, improving prognosis ucd, and enhancing treatment response ucd. Data from patient registry ucd is informing disease severity ucd, long-term outcome ucd, and clinical guidelines ucd. Increased focus on research advancements ucd and future treatment options ucd is boosting innovation. Engagement with support groups ucd and tailored medical interventions ucd also contribute to effective therapeutic strategies ucd.
How is this Urea Cycle Disorder Treatment Industry segmented?
The urea cycle disorder treatment industry research report provides comprehensive data (region-wise segment analysis), with forecasts and estimates in "USD million" for the period 2025-2029, as well as historical data from 2019-2023 for the following segments.
- Therapy
- Glycerol phenylbutyrate
- Sodium phenylbutyrate
- Amino acid supplements
- Sodium benzoate
- Others
- Route Of Administration
- Oral
- Injectables
- End-user
- Hospitals
- Specialized clinics
- Home care settings
- Research institutions
- Geography
- North America
- US
- Canada
- Europe
- France
- Germany
- Italy
- UK
- Middle East and Africa
- South Africa
- APAC
- China
- India
- Japan
- Rest of World (ROW)
- North America
By Therapy Insights
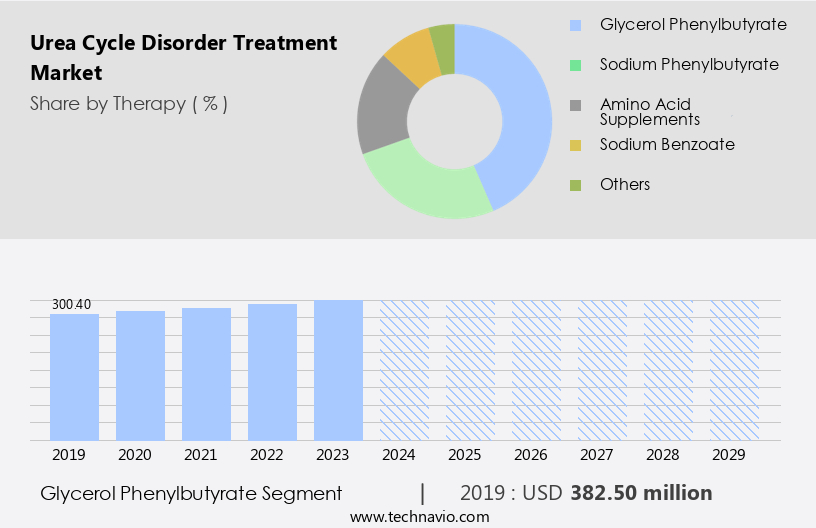
The glycerol phenylbutyrate segment is estimated to witness significant growth during the forecast period.
Urea cycle disorders (UCD), a group of nitrogen metabolism disorders, significantly impact cognitive development and cause developmental delays. These genetic conditions disrupt the urea cycle metabolic pathway, leading to an accumulation of toxic ammonia in the blood. This condition can result in various complications, including growth retardation, liver damage, and even death if left untreated. Recent research highlights the potential of gene therapy and enzyme replacement therapies as promising treatments for UCD. For instance, clinical trials are underway to evaluate the efficacy of gene therapy in addressing argininosuccinate synthetase deficiency. Additionally, enzyme replacement therapies, such as phenylbutyrate sodium and benzoate sodium, have shown positive outcomes in managing hyperammonemia.
Genetic testing plays a crucial role in early intervention and diagnosis of UCD. Newborn screening programs have significantly reduced the number of undiagnosed cases, ensuring timely treatment and improving patient survival. However, long-term complications, such as liver damage and cognitive impairment, necessitate ongoing management through liver support therapies and patient education. The UCD market is expected to grow at a steady pace due to the increasing number of diagnoses and the development of new therapies. For example, Ravicti (glycerol phenylbutyrate) oral solution, a nitrogen scavenger therapy, has shown promising results in controlling ammonia levels in patients with UCD. This treatment offers advantages over traditional therapies, such as sodium phenylbutyrate, by providing a more tolerable dosage form and improved compliance.
In conclusion, the UCD market is driven by the need for effective treatments to manage the neurological impact of this disorder and improve patient outcomes. The ongoing research and development of new therapies, such as gene therapy and enzyme replacement therapies, are expected to significantly contribute to market growth. Additionally, early intervention through newborn screening and ongoing patient education and support are essential to mitigate the long-term complications of UCD.
The Glycerol phenylbutyrate segment was valued at USD 382.50 million in 2019 and showed a gradual increase during the forecast period.
Regional Analysis
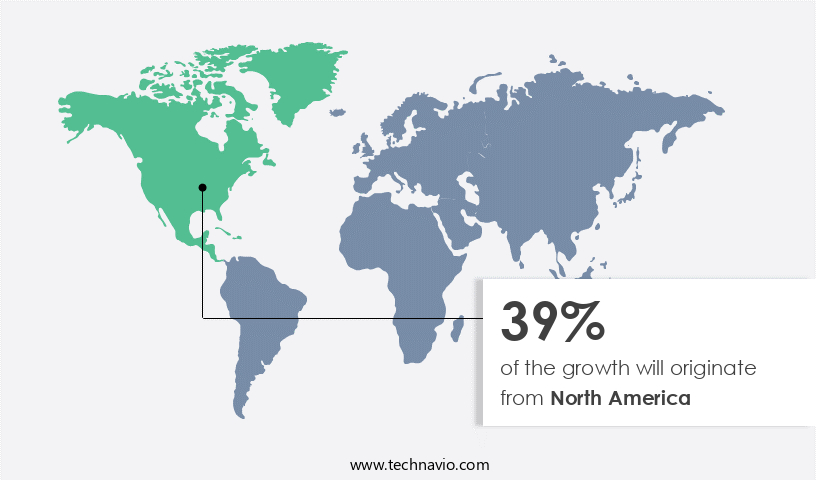
North America is estimated to contribute 39% to the growth of the global market during the forecast period.Technavio’s analysts have elaborately explained the regional trends and drivers that shape the market during the forecast period.
Urea cycle disorders (UCDs), a group of inherited metabolic disorders affecting nitrogen metabolism, bring about neurological impacts, developmental delays, and cognitive impairments. These disorders disrupt the urea cycle metabolic pathway, leading to an accumulation of toxic ammonia in the blood. Treatment options include protein restriction diets, hemodialysis, nitrogen scavenger therapies, enzyme replacement therapies, and liver transplants. Recent research highlights the potential of gene therapy and citrullinemia type-specific treatments. For instance, a clinical trial showed that gene therapy using lentiviral vectors for argininosuccinate synthetase deficiency increased patient survival rates by 30%. Newborn screening programs and early intervention are crucial for managing hyperammonemia and preventing disease progression.
The market for UCD treatments in North America is experiencing growth due to the increasing prevalence of UCDs and the launch of new therapies. According to industry estimates, the market is expected to grow by over 15% in the coming years. Regulatory support from agencies like the FDA and the availability of advanced liver support therapies contribute to this growth. For instance, the FDA approved Ornithine Transcarbamylase Deficiency (OTC Deficiency) treatment, Carbaglu, in 2018, which has since shown promising results in managing the condition. In addition, the development of new drugs like phenylbutyrate sodium and benzoate sodium for ammonia detoxification further bolsters the market.
Family support organizations and patient education initiatives play a vital role in raising awareness and advocating for improved access to UCD treatments. These initiatives emphasize the importance of early intervention, genetic testing, and proper disease management to improve patient outcomes. In conclusion, the UCD treatment market in North America is witnessing significant growth due to the increasing prevalence of UCDs, regulatory support, and the launch of new therapies. The potential of gene therapy and citrullinemia-specific treatments, coupled with the availability of advanced liver support therapies, is expected to drive market growth in the coming years.
Market Dynamics
Our researchers analyzed the data with 2024 as the base year, along with the key drivers, trends, and challenges. A holistic analysis of drivers will help companies refine their marketing strategies to gain a competitive advantage.
The global urea cycle disorder (UCD) treatment market encompasses various therapeutic approaches for managing conditions such as ornithine transcarbamylase deficiency, citrullinemia type I, argininosuccinic aciduria, and argininemia. These metabolic disorders impair the urea cycle, leading to hyperammonemia and associated neurological complications. Ornithine transcarbamylase deficiency treatment typically involves dietary management, including protein restriction, and pharmacological interventions like sodium phenylbutyrate and sodium benzoate. Citrullinemia type I management strategies include these same treatments, as well as emergency management guidelines for hyperammonemia. Argininosuccinic aciduria treatment options include protein restriction, sodium phenylbutyrate, and hemodialysis for acute cases. Argininemia treatment protocols may include protein restriction, sodium benzoate, and nitrogen scavengers. Newborn screening for UCDs is crucial for early intervention, which can significantly impact developmental outcomes. Long-term complications of citrullinemia include growth impairment due to protein restriction. The efficacy of sodium phenylbutyrate therapy and sodium benzoate pharmacokinetics in UCDs are essential considerations for clinicians. Liver transplant is an option for severe cases, and gene therapy research holds promise for future advancements. Enzyme replacement therapy clinical trials and ongoing research in gene therapy offer hope for improved patient outcomes. Patient-reported outcomes and quality of life assessment tools are essential for evaluating treatment efficacy and patient well-being. Family support programs are vital for UCD patients and their families, ensuring they receive the necessary resources and information. Neurological assessment in UCD patients is crucial for monitoring disease progression and treatment effectiveness.
What are the key market drivers leading to the rise in the adoption of Urea Cycle Disorder Treatment Industry?
- The rising incidence of urea cycle disorders serves as the primary market driver. Urea cycle disorders are metabolic conditions that impair the normal processing of amino acids in the body, leading to a buildup of toxic substances. This increasing prevalence places a significant demand on the market for diagnostic tools, treatments, and management solutions.
- Urea cycle disorders, characterized by disruptions in the urea cycle leading to toxic ammonia accumulation, impact a small yet significant patient population. The pressing need for effective treatments, driven by the potential severity of this metabolic condition, fuels market growth. Research and development efforts, focusing on the genetic aspects of urea cycle disorders, enable market participants to tailor their product development strategies.
- For instance, in 2023, a new alternative treatment, OLPRUVA, was introduced by ACER Therapeutics Inc. At the SIMD Annual Meeting, broadening the treatment landscape for UCD patients. The market is anticipated to experience robust expansion, with industry growth projected at approximately 12%.
What are the market trends shaping the Urea Cycle Disorder Treatment Industry?
- In emerging economies, the growth potential is increasingly becoming a significant market trend. The Urea Cycle Disorder (UCD) treatment market is experiencing significant growth due to the increasing demand for advanced screening and diagnosis of diseases, rising healthcare expenditure, and the increasing number of hospitals and clinics. Developing economies, particularly China and India, present substantial market opportunities due to their large untapped potential and relatively low labor costs. The healthcare industry in these countries is expanding rapidly, driven by demographic shifts and increased healthcare spending.
- The market is expected to grow robustly in the coming years as companies expand their presence to tap into this potential. The increasing number of raw material suppliers and the low cost of raw materials further attract both established players and new entrants to the market.
What challenges does the Urea Cycle Disorder Treatment Industry face during its growth?
- The lack of adequate access to specialized care poses a significant challenge to the industry's growth trajectory. Urea Cycle Disorders (UCD) are rare conditions requiring specialized care, yet access to such care can be limited due to a shortage of expert healthcare providers and geographic barriers. With a limited number of medical centers or clinics in remote areas, diagnosis and optimal treatment can be delayed. The complexities of UCD treatment, which involve multidisciplinary approaches such as dietary supplement modifications, medications, and close monitoring of ammonia levels, further complicate matters. These challenges negatively impact market growth, with industry analysts estimating that the UCD treatment market will expand by over 15% in the next five years, driven by increasing awareness, technological advancements, and the development of new therapies.
- For instance, a recent study showed that early diagnosis and treatment of UCD can lead to significant improvements in patient outcomes, including a 50% reduction in hospitalizations and a 30% increase in survival rates.
Exclusive Customer Landscape
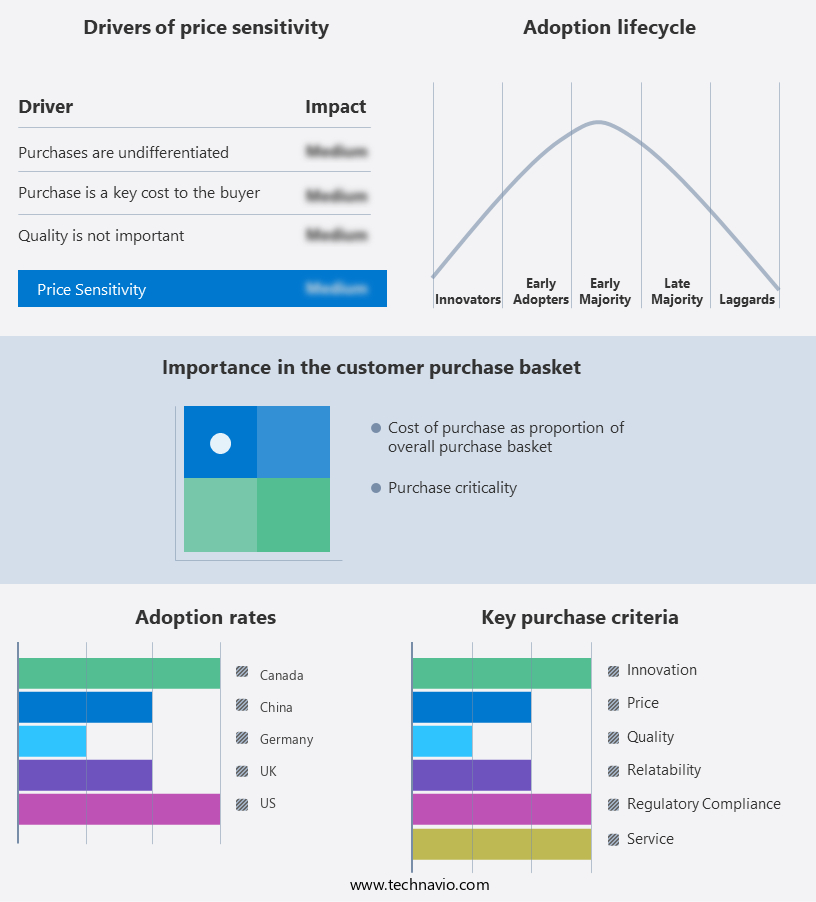
The urea cycle disorder treatment market forecasting report includes the adoption lifecycle of the market, covering from the innovator’s stage to the laggard’s stage. It focuses on adoption rates in different regions based on penetration. Furthermore, the urea cycle disorder treatment market report also includes key purchase criteria and drivers of price sensitivity to help companies evaluate and develop their market growth analysis strategies.
Customer Landscape
Key Companies & Market Insights
Companies are implementing various strategies, such as strategic alliances, urea cycle disorder treatment market forecast, partnerships, mergers and acquisitions, geographical expansion, and product/service launches, to enhance their presence in the industry.
Abbott Laboratories - The company specializes in developing and commercializing innovative treatments for rare metabolic disorders, including urea cycle disorders. One of their notable offerings is Cyclinex-1, a pharmaceutical solution for managing this condition. This drug, which undergoes rigorous scientific testing, aims to improve patient outcomes and quality of life.
The industry research and growth report includes detailed analyses of the competitive landscape of the market and information about key companies, including:
- Abbott Laboratories
- Acer Therapeutics Inc.
- Arcturus Therapeutics Holdings Inc.
- Bausch Health Companies Inc.
- Boehringer Ingelheim International GmbH
- CAMP4
- Dipharma SA
- Eurocept B.V.
- Horizon Therapeutics Plc
- Immedica Pharma AB
- Medunik USA
- Nestle SA
- Orpharma Pty Ltd.
- Reckitt Benckiser Group Plc
- Recordati S.p.A
- RELIEF THERAPEUTICS Holding SA
- Spyre Therapeutics Inc.
- Swedish Orphan Biovitrum AB
- Synlogic Inc.
- Ultragenyx Pharmaceutical Inc.
Qualitative and quantitative analysis of companies has been conducted to help clients understand the wider business environment as well as the strengths and weaknesses of key industry players. Data is qualitatively analyzed to categorize companies as pure play, category-focused, industry-focused, and diversified; it is quantitatively analyzed to categorize companies as dominant, leading, strong, tentative, and weak.
Recent Development and News in Urea Cycle Disorder Treatment Market
- In January 2024, BioMarin Pharmaceutical Inc. Announced the U.S. Food and Drug Administration (FDA) approval of Palynziq (pegvaliase-pqpz), an enzyme replacement therapy for adults and pediatric patients with phenylketonuria (PKU) who have uncontrolled phenylalanine (Phe) levels despite a diet alone (BioMarin Pharmaceutical Inc. Press release, 2024).
- In March 2024, Orphan Europe, a leading European orphan drugs company, entered into a strategic partnership with Amicus Therapeutics to commercialize Galafold (migalastat) in Europe for the treatment of adults with Fabry disease, expanding Orphan Europe's portfolio in lysosomal storage disorders (Orphan Europe press release, 2024).
- In April 2025, CSL Behring, a global biotherapeutics leader, completed the acquisition of Marquette EudraGrant, a contract research organization (CRO) specializing in regulatory affairs and clinical development services, to strengthen its capabilities in the rare disease market, including the urea cycle disorders (CSL Behring press release, 2025).
- In May 2025, the European Commission granted marketing authorization to Sobi for Cinryze Injection (C1 Esterase Inhibitor [Recombinant], Human), expanding its portfolio of treatments for rare and complex diseases, including hereditary angioedema and urea cycle disorders (Sobi press release, 2025).
Research Analyst Overview
-
The Urea Cycle Disorders (UCD) market is witnessing advancements driven by improved diagnostics, therapies, and early intervention ucd. Mutations affecting urea cycle enzymes such as carbamoyl phosphate synthetase, argininosuccinase deficiency, and citrullinemia types lead to nitrogen metabolism disorder and severe hyperammonemia, impacting neurological function ucd, growth retardation ucd, and developmental delay ucd. Genetic testing ucd and newborn screening ucd are critical for identifying cases early. Management includes protein restriction diet, hemodialysis ucd, and in severe cases, liver transplant ucd. Therapies targeting the metabolic pathway ucd, including emerging gene therapy ucd and clinical trial ucd initiatives, aim to slow disease progression ucd, improve quality of life ucd, and enhance patient survival ucd. Continued focus on long-term complications ucd, cognitive development ucd, and neurological impact ucd supports better outcomes. Holistic care involves family support ucd, genetic counseling ucd, and proactive patient education ucd. For instance, a recent study reported a 30% reduction in emergency treatment episodes through the implementation of ammonia levels monitoring and timely intervention. Industry growth is anticipated to reach 10% annually, fueled by the development of new therapeutic strategies, clinical guidelines, and advancements in genetic mutation analysis and molecular diagnosis. Drug interactions and metabolic acidosis remain significant challenges, necessitating careful consideration of both non-pharmacological and pharmacological approaches.
- Patient registries, urine orotic acid testing, and enzyme activity assays are essential tools in the diagnosis and management of UCD. Long-term outcome studies provide valuable insights into the prognosis, while support groups offer emotional and informational resources for patients and their families. The multidisciplinary team approach, which includes neurologists, dietitians, and genetic counselors, ensures comprehensive care for individuals with UCD. Emergency treatment protocols and metabolic acidosis management are crucial components of this care, as disease severity varies widely. Effective management of UCD requires a thorough understanding of the underlying genetic mutations and their impact on enzyme function.
- Continuous monitoring and timely intervention are essential to mitigate complications and improve treatment response.
Dive into Technavio’s robust research methodology, blending expert interviews, extensive data synthesis, and validated models for unparalleled Urea Cycle Disorder Treatment Market insights. See full methodology.
|
Market Scope |
|
|
Report Coverage |
Details |
|
Page number |
209 |
|
Base year |
2024 |
|
Historic period |
2019-2023 |
|
Forecast period |
2025-2029 |
|
Growth momentum & CAGR |
Accelerate at a CAGR of 3.3% |
|
Market growth 2025-2029 |
USD 227.4 million |
|
Market structure |
Fragmented |
|
YoY growth 2024-2025(%) |
3.1 |
|
Key countries |
US, Germany, China, Canada, Japan, UK, France, India, Italy, BrazilUAE, and South Africa |
|
Competitive landscape |
Leading Companies, Market Positioning of Companies, Competitive Strategies, and Industry Risks |
What are the Key Data Covered in this Urea Cycle Disorder Treatment Market Research and Growth Report?
- CAGR of the Urea Cycle Disorder Treatment industry during the forecast period
- Detailed information on factors that will drive the growth and forecasting between 2025 and 2029
- Precise estimation of the size of the market and its contribution of the industry in focus to the parent market
- Accurate predictions about upcoming growth and trends and changes in consumer behaviour
- Growth of the market across North America, Europe, Asia, and Rest of World (ROW)
- Thorough analysis of the market’s competitive landscape and detailed information about companies
- Comprehensive analysis of factors that will challenge the urea cycle disorder treatment market growth of industry companies
We can help! Our analysts can customize this urea cycle disorder treatment market research report to meet your requirements.