US Active Pharmaceutical Ingredient Market Size 2025-2029
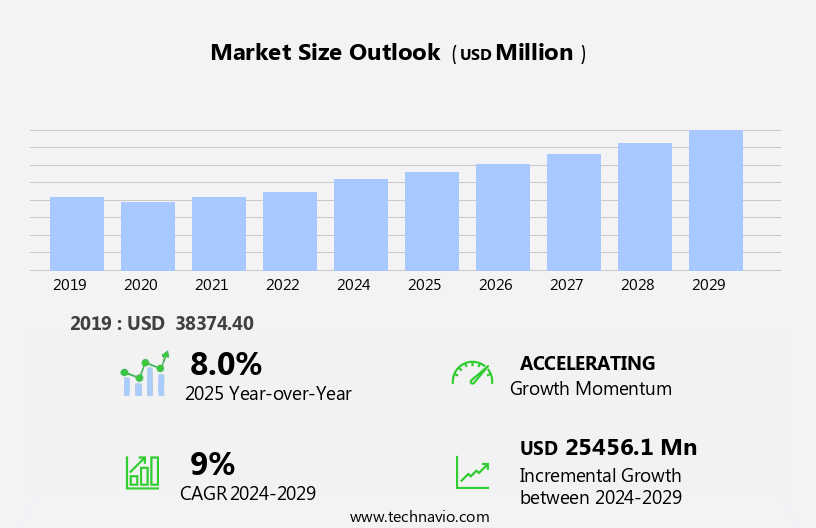
The US active pharmaceutical ingredient (API) market size is forecast to increase by USD 25.46 billion at a CAGR of 9% between 2024 and 2029.
- The Active Pharmaceutical Ingredient (API) market is experiencing significant growth, driven by the increasing number of Type II Drug Master Files (DMFs) and a paradigm shift towards contract manufacturing organizations (CMOs) for API production. This trend is a response to the complexities and costs associated with in-house API manufacturing. However, market expansion is not without challenges. Regulatory hurdles impact adoption, as stringent regulations necessitate extensive compliance measures. Furthermore, supply chain inconsistencies temper growth potential due to the reliance on multiple suppliers and the need for high-quality raw materials. High investment costs and the risk of substantial losses due to production failures or regulatory non-compliance add to the complexity of the market landscape. One major factor is the increasing prevalence of hospital-acquired infections, leading to a higher demand for APIs used in antibiotics.
- Companies seeking to cAPItalize on market opportunities must navigate these challenges effectively, focusing on regulatory compliance, supply chain transparency, and risk mitigation strategies. By doing so, they can position themselves as trusted partners to pharmaceutical and biotech companies, ensuring long-term success in the evolving API market. APIs play a crucial role in the development of targeted therapies, which are designed to address specific genetic mutations or biomarkers.
What will be the size of the US Active Pharmaceutical Ingredient (API) Market during the forecast period?
- The Active Pharmaceutical Ingredient (API) market is characterized by ongoing advancements in technology and regulatory requirements. Personalized medicine and precision medicine are driving the need for advanced bioanalytical methods, such as mass spectrometry, to ensure accurate drug dosing and individualized treatment plans. Simultaneously, drug shortages and withdrawals necessitate process optimization and real-time release testing to mitigate supply chain disruptions. Pharmacodynamic and pharmacokinetic studies employ in vivo and in vitro techniques to understand drug behavior and interaction with the body. Microfluidic technology and flow chemistry facilitate process optimization and continuous manufacturing, enhancing efficiency and reducing waste. Pharmaceutical regulations, including quality by design and process validation, ensure drug safety and adverse event monitoring. This market is driven by several factors, including the rising prevalence of chronic diseases, the demand for biologics and biosimilars, and the increasing focus on precision medicine
- Green chemistry and analytical method development contribute to the reduction of harmful by-products and the creation of more sustainable manufacturing processes. Pharmaceutical legislation addresses drug diversion, counterfeiting, and clinical trial design, ensuring transparency and patient protection. Drug interactions and adverse events necessitate rigorous analytical method validation and process analytical technology to ensure drug efficacy and safety. The increasing number of DMF filings with the US Food and Drug Administration (FDA) is one such factor, as these filings provide essential information for the manufacturing, processing, and packaging of drugs for human use.
How is this market segmented?
The market research report provides comprehensive data (region-wise segment analysis), with forecasts and estimates in "USD million" for the period 2025-2029, as well as historical data from 2019-2023 for the following segments.
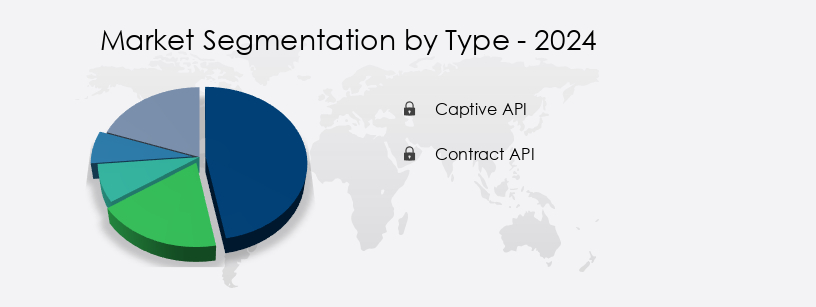
- Type
- Captive API
- Contract API
- Product
- Generic
- Innovative
- Application
- Oncology
- Cardiovascular diseases
- Diabetes
- Communicable diseases
- Others
- Geography
- North America
- US
- North America
By Type Insights
The captive API segment is estimated to witness significant growth during the forecast period. The active pharmaceutical ingredient (API) market in the US is experiencing notable growth due to the increasing emphasis on affordable healthcare services. This trend is driving demand for low-cost APIs, leading pharmaceutical companies to outsource the manufacturing of bulk actives and late-stage intermediates to contract manufacturing organizations (CMOs). This shift has resulted in a decline in the market share of captive API manufacturing. The biopharmaceutical sector, including biologics and biosimilars, is also contributing to the growth of the API market. Biotechnology companies, pharmaceutical outsourcing firms, and contract research organizations are key players in this market, providing services such as custom synthesis, formulation development, clinical trial management, and regulatory compliance. The therapeutic areas of infectious disease, drug delivery systems, and controlled release are major focus areas for API development.
Get a glance at the market share of various segments Request Free Sample
The Captive API segment was valued at USD 21201.90 million in 2019 and showed a gradual increase during the forecast period. Initially focusing on early-stage API intermediates, outsourcing has since expanded to advanced intermediates and final dosage forms, particularly for generic drugs. Biotech startups and specialty pharmaceutical companies are also significant contributors to the market, leveraging advanced technologies like chemical synthesis, pharmaceutical licensing, and process chemistry for API production. Intellectual property protection and pricing strategies are crucial considerations in the pharmaceutical supply chain, ensuring regulatory compliance and ensuring the quality of APIs. The pharmaceutical industry's focus on drug discovery, clinical trials, and pharmaceutical sales is further fueling the demand for APIs. Overall, the API market in the US is a dynamic and evolving landscape, driven by the interplay of various entities and market forces. The market has witnessed significant changes in recent decades, with many pharmaceutical companies outsourcing their research and manufacturing processes to Contract Manufacturing Organizations (CMOs) and other drug manufacturing entities.
Market Dynamics
Our researchers analyzed the data with 2024 as the base year, along with the key drivers, trends, and challenges. A holistic analysis of drivers will help companies refine their marketing strategies to gain a competitive advantage.
What are the key market drivers leading to the rise in the adoption of Active Pharmaceutical Ingredient (API) in US Industry?
- The rising prevalence of type II drugs, reflected in the growing number of Drug Master Files (DMFs) for these compounds, is the primary market driver. The Active Pharmaceutical Ingredient (API) market in the US is experiencing significant growth due to several factors. The increasing healthcare expenditure by the urban population and the aging population are key drivers. Additionally, the filing of Drug Master Files (DMFs) with the US Food and Drug Administration (FDA) is contributing to market expansion. A DMF provides detailed information about the facilities, processes, and materials used in the manufacturing, processing, and packaging of drugs for human use. This information can support various applications, including Investigational New Drug (IND), New Drug Application (NDA), Abbreviated New Drug Application (ANDA), and export applications.
- Quality control, regulatory compliance, and regulatory affairs are essential aspects of the pharmaceutical industry, making the API market a critical component. Pharmaceutical companies, including biotechnology firms, are increasingly outsourcing pharmaceutical engineering, consulting, and contract manufacturing services to ensure regulatory compliance and maintain focus on their core competencies.
What are the market trends shAPIng the Active Pharmaceutical Ingredient (API) in US Industry?
- The trend in API manufacturing is shifting towards new paradigms. This mandatory transition reflects the evolving market demands. The Active Pharmaceutical Ingredient (API) market in the US has undergone significant transformation in recent decades, with many pharmaceutical companies opting to outsource their research and manufacturing processes to Contract Research Organizations (CMOs) and other drug manufacturing entities. Initially, outsourcing was limited to early-stage API intermediates. However, it has since expanded across the value chain, encompassing advanced intermediates and final dosage forms for both branded and generic drugs. The decision to outsource an API is influenced by various factors, including industry trends, the cost of acquiring new, in-house technologies, and the availability of internal capacity. This shift towards outsourcing is particularly prevalent in the generic drug sector, where cost savings and efficiency are key drivers.
- Moreover, the API market caters to various therapeutic areas, including infectious diseases, oncology, and neurology, among others. Formulation development, clinical trial management, and drug delivery systems, such as controlled release and biodegradable polymers, are essential components of the API market. Biotech startups also contribute significantly to the market, driving innovation and competition. In summary, the API market in the US has witnessed a notable shift towards outsourcing, with CMOs and other drug manufacturing organizations playing a crucial role in supplying APIs for various pharmaceutical applications. This trend is expected to continue, driven by cost savings, efficiency, and the need for innovation in the pharmaceutical industry.
What challenges does the Active Pharmaceutical Ingredient (API) in US Industry face during its growth?
- The high investment costs and potential for significant losses pose a significant challenge to the industry's growth trajectory. Active Pharmaceutical Ingredients (APIs) are essential components of pharmaceutical products. The production of APIs involves chemical synthesis and adherence to stringent regulatory standards, which necessitates substantial investment in infrastructure, technology, and compliance. The high regulatory compliance costs, including testing, validation, and continuous monitoring, contribute to the overall investment burden. Moreover, the complex nature of pharmaceutical research and development, which includes extensive testing and clinical trials, adds to the cost structure. These factors create barriers for new entrants and even challenge existing companies to expand their API production capacity. Despite these challenges, the demand for APIs continues to grow due to the increasing need for pharmaceutical products.
- Effective supply chain management plays a crucial role in ensuring the timely delivery of APIs to pharmaceutical manufacturers, enabling them to meet their production schedules and maintain their pharmaceutical sales. The API market is characterized by significant investment requirements, stringent regulatory standards, and a complex research and development process. These market dynamics necessitate strategic planning and a robust business model for companies looking to succeed in this industry.
Exclusive Customer Landscape
The active pharmaceutical ingredient (API) market in US forecasting report includes the adoption lifecycle of the market, covering from the innovator's stage to the laggard's stage. It focuses on adoption rates in different regions based on penetration. Furthermore, the active pharmaceutical ingredient (API) market in US report also includes key purchase criteria and drivers of price sensitivity to help companies evaluate and develop their market growth analysis strategies.
Customer Landscape
Key Companies & Market Insights
Companies are implementing various strategies, such as strategic alliances, active pharmaceutical ingredient (API) market in US forecast, partnerships, mergers and acquisitions, geographical expansion, and product/service launches, to enhance their presence in the industry.
AbbVie Inc. - The company specializes in the production of active pharmaceutical ingredients (APIs), including Biperiden, Cyclosporine, Dasabuvir, organometallic, and gaseous HCl.
The industry research and growth report includes detailed analyses of the competitive landscape of the market and information about key companies, including:
- AbbVie Inc.
- Amneal Pharmaceuticals Inc.
- Apotex Inc.
- Aurobindo Pharma Ltd.
- Bristol Myers Squibb Co.
- Cadila Pharmaceuticals Ltd.
- Cambrex Corp.
- Cipla Inc.
- Dr Reddys Laboratories Ltd.
- DSM-Firmenich AG
- GlaxoSmithKline Plc
- Lupin Ltd.
- Merck KGaA
- Novartis AG
- Pfizer Inc.
- Sanofi SA
- Sun Pharmaceutical Industries Ltd.
- Teva Pharmaceutical Industries Ltd.
- Thermo Fisher Scientific Inc.
- Viatris Inc.
Qualitative and quantitative analysis of companies has been conducted to help clients understand the wider business environment as well as the strengths and weaknesses of key industry players. Data is qualitatively analyzed to categorize companies as pure play, category-focused, industry-focused, and diversified; it is quantitatively analyzed to categorize companies as dominant, leading, strong, tentative, and weak.
Recent Development and News in Active Pharmaceutical Ingredient (API) Market In US
- In February 2024, Merck KGaA and Parexel International Corporation announced a strategic collaboration to enhance the development and manufacturing of APIs for the biopharmaceutical industry. This partnership aimed to streamline the drug development process and improve efficiency, as reported by Merck KGaA's press release (Merck KGaA, 2024).
- In June 2024, DSM and Mylan N.V. Announced the completion of their merger, creating a leading global supplier of APIs and generic drugs. This merger expanded DSM's presence in the pharmaceutical industry and strengthened Mylan's API production capabilities, according to DSM's press release (DSM, 2024)
Research Analyst Overview
The active pharmaceutical ingredient (API) market continues to evolve, driven by the dynamic interplay of various sectors and entities. Chemical synthesis plays a pivotal role in API production, with ongoing advancements shAPIng the landscape. Pharmaceutical licensing and API synthesis are integral components of this process, with licensing agreements facilitating the transfer of intellectual property and synthesis enabling the production of complex APIs. Pharmaceutical manufacturing and supply chain management are also key areas of focus, as companies strive to optimize production and distribution processes. Clinical trials and drug discovery are inextricably linked to the API market, with new therAPIes driving demand for novel APIs and bulk drug substances. API manufacturing involves chemical processes and protein synthesis, with advancements in artificial intelligence and expression systems (yeast, insect, and other systems) streamlining production. T
Pharmaceutical sales and pharmaceutical intermediates are additional facets of the market, with pricing strategies and regulatory compliance playing crucial roles in shAPIng market dynamics. The therapeutic area focus shifts as the industry responds to emerging health challenges, with a growing emphasis on infectious diseases and specialized therapeutics. Biotech startups and pharmaceutical outsourcing are transforming the industry, with contract manufacturing and pharmaceutical consulting providing valuable services to companies seeking to bring new drugs to market. Drug delivery systems, including controlled release and therapeutic area-specific formulations, are also driving innovation in the API market. Process chemistry and organic chemistry continue to advance, with analytical services playing a critical role in ensuring product quality and regulatory compliance. Intellectual property and contract research organizations are essential players in the API market, providing critical support in the development and commercialization of new drugs. The market caters to generics and various therapeutic segments, including cardiology, with the World Heart Report highlighting CVD-related deaths and the importance of statins like simvastatin and rosuvastatin calcium. The API market is a complex and ever-evolving ecosystem, with ongoing activity and emerging patterns shAPIng its future.
Dive into Technavio's robust research methodology, blending expert interviews, extensive data synthesis, and validated models for unparalleled Active Pharmaceutical Ingredient (API) Market in US insights. See full methodology.
|
Market Scope |
|
|
Report Coverage |
Details |
|
Page number |
177 |
|
Base year |
2024 |
|
Historic period |
2019-2023 |
|
Forecast period |
2025-2029 |
|
Growth momentum & CAGR |
Accelerate at a CAGR of 9% |
|
Market growth 2025-2029 |
USD 25.46 billion |
|
Market structure |
Fragmented |
|
YoY growth 2024-2025(%) |
8.0 |
|
Competitive landscape |
Leading Companies, Market Positioning of Companies, Competitive Strategies, and Industry Risks, |
What are the Key Data Covered in this Active Pharmaceutical Ingredient (API) Market in US Research and Growth Report?
- CAGR of the Active Pharmaceutical Ingredient (API) in US industry during the forecast period
- Detailed information on factors that will drive the growth and forecasting between 2025 and 2029
- Precise estimation of the size of the market and its contribution of the industry in focus to the parent market
- Accurate predictions about upcoming growth and trends and changes in consumer behaviour
- Growth of the market across US
- Thorough analysis of the market's competitive landscape and detailed information about companies
- Comprehensive analysis of factors that will challenge the active pharmaceutical ingredient (API) market in US growth of industry companies
We can help! Our analysts can customize this active pharmaceutical ingredient (API) market in US research report to meet your requirements.