Emergency Contraceptive Pills Market Size 2025-2029
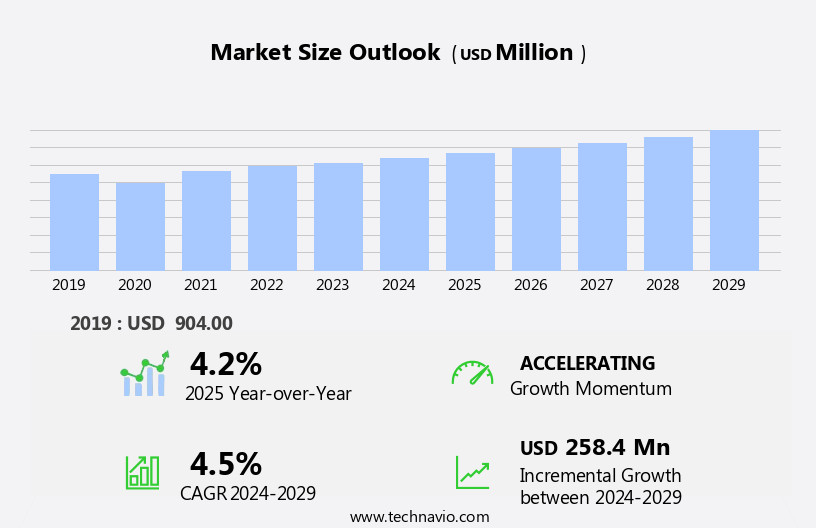
The emergency contraceptive pills market size is forecast to increase by USD 258.4 million, at a CAGR of 4.5% between 2024 and 2029.
- The Emergency Contraceptive Pills (ECP) market is driven by a rising number of initiatives to create awareness about family planning and the importance of preventing unintended pregnancies. This trend is particularly significant in regions where access to regular contraceptive methods may be limited or ineffective. However, market growth is challenged by local governments limiting access to ECPs due to cultural, religious, or political reasons. These restrictions can hinder market expansion and limit the reach of these essential health products to those who need them most.
- Companies operating in the ECP market must navigate these regulatory complexities while continuing to invest in awareness campaigns and product innovation to meet the evolving needs of consumers. Effective strategies will include collaborating with local organizations, advocacy groups, and healthcare providers to expand access and ensure affordability, ultimately contributing to improved reproductive health outcomes and gender equality.
What will be the Size of the Emergency Contraceptive Pills Market during the forecast period?
Explore in-depth regional segment analysis with market size data - historical 2019-2023 and forecasts 2025-2029 - in the full report.
Request Free Sample
The market continues to evolve, influenced by various factors in the global health sector. Distribution channels expand as online pharmacies and retail outlets increase accessibility. Health Insurance coverage remains a critical consideration, with ongoing negotiations impacting patient access. Drug interactions and adverse effects necessitate continual safety studies and regulatory approval. Marketing and promotion strategies adapt to reach diverse demographics, including public health initiatives and sexual health education. Reproductive health remains a priority, with contraceptive counseling and patient education integral to effective use. Clinical trials explore new dosage forms and mechanisms, such as implantation prevention and ovulation inhibition. Access to healthcare is a continual focus, with efforts to expand availability and reduce barriers.
Drug development advances with the introduction of generic drugs and alternative contraceptive options, such as the copper IUD. FDA approval processes and prescription requirements ensure safety and efficacy. Social media marketing and patient education campaigns raise awareness and promote safe sex practices. Ongoing research addresses unintended pregnancy prevention and HIV prevention. Pricing strategies and over-the-counter availability continue to shape market dynamics. Healthcare providers play a crucial role in emergency contraception, offering counseling and administering medications. Pharmaceutical manufacturing and regulatory bodies work to ensure quality and safety. Oral administration remains the primary method, but new technologies and dosage forms are under investigation.
Emergency contraception effectiveness is a continuous area of study, with ongoing efficacy and safety studies. Adverse effects and side effects are closely monitored, with efforts to minimize risks. The market's evolution reflects the ongoing commitment to women's health and reproductive rights.
How is this Emergency Contraceptive Pills Industry segmented?
The emergency contraceptive pills industry research report provides comprehensive data (region-wise segment analysis), with forecasts and estimates in "USD million" for the period 2025-2029, as well as historical data from 2019-2023 for the following segments.
- Distribution Channel
- Retail stores
- Online stores
- Type
- Combination pills
- Progesterone pills
- Geography
- North America
- US
- Canada
- Europe
- France
- Germany
- Italy
- UK
- APAC
- China
- India
- Japan
- South America
- Brazil
- Rest of World (ROW)
- North America
.
By Distribution Channel Insights
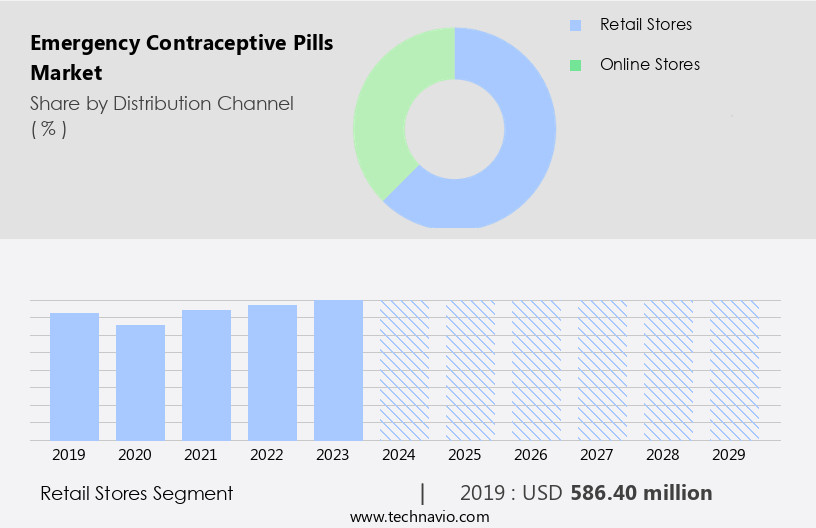
The retail stores segment is estimated to witness significant growth during the forecast period.
In the realm of healthcare, the market for emergency contraceptive pills (ECPs) continues to evolve, with various entities shaping its dynamics. Retail stores, including supermarkets, hypermarkets, departmental stores, pharmacies and clinics, and other retail outlets, serve as significant distribution channels for these medications. Brands like Plan B One-Step by Foundation Consumer Healthcare are available at retailers such as Walmart, Walgreens, and CVS Pharmacy. Retailers focus on enhancing the consumer experience in purchasing ECPs by ensuring knowledgeable sales staff, inspiring trust and confidence in the buying process. This trend benefits the global market for emergency contraceptive pills. The rise in disposable income and the availability of these pills across diverse retail stores contribute to their convenience for consumers.
Global health organizations and public health initiatives promote the use of ECPs for unintended pregnancy prevention and HIV prevention. Health insurance coverage for these medications is increasingly common, expanding access to healthcare. New Drug development companies are engaged in clinical trials to improve ECP efficacy, safety, and dosage forms, including copper IUDs and implantation prevention. Pharmaceutical manufacturing companies, such as those producing ulipristal acetate, are involved in the development and FDA approval of new ECPs. Healthcare providers play a crucial role in contraceptive counseling and prescription requirements, while regulatory approval processes ensure safety and efficacy. Marketing and promotion strategies, including social media marketing, public awareness campaigns, and patient education, are employed to increase access to ECPs.
Next-generation ECPs, such as generic drugs, are entering the market, offering affordable alternatives. Reproductive rights organizations advocate for over-the-counter availability of ECPs, improving women's health and reducing unintended pregnancies. Safety studies and side effects are under constant scrutiny, with pharmaceutical companies and regulatory bodies collaborating to minimize adverse effects. Sexual health education and safe sex practices are essential components of family planning, alongside the use of hormonal contraception and emergency contraceptive pills. In summary, the market is influenced by various entities, including retailers, global health organizations, drug development companies, healthcare providers, regulatory bodies, and advocacy groups. These entities collaborate to ensure access to effective and safe contraceptive options, promote public awareness, and improve women's health.
The Retail stores segment was valued at USD 586.40 million in 2019 and showed a gradual increase during the forecast period.
Regional Analysis
North America is estimated to contribute 45% to the growth of the global market during the forecast period.Technavio's analysts have elaborately explained the regional trends and drivers that shape the market during the forecast period.
The market in North America is experiencing significant growth due to increased product awareness and accessibility. These pills are available at pharmacies, clinics, and online pharmacies, making them convenient for users. The market's expansion is further fueled by the high incidence of unintended pregnancies, which reached approximately 42% in the US in 2023. This alarming figure highlights the importance and benefits of emergency contraceptive pills, leading to increased demand. However, the market's growth is slightly hindered by incorrect usage, with some users taking incorrect dosages or consuming pills beyond the recommended 72-hour window, rendering them ineffective.
Global health initiatives, healthcare providers, and public health campaigns are promoting the use of emergency contraceptive pills as a crucial component of family planning and reproductive health. Drug development companies are focusing on creating various dosage forms, including hormonal contraceptives like Plan B One-Step and ulipristal acetate, as well as non-hormonal options like the copper IUD and implantation prevention. Marketing and promotion efforts include social media campaigns, patient education, and prescription requirements. Regulatory approval and safety studies are crucial in ensuring the efficacy and safety of these pills, with the FDA playing a significant role in their approval.
Adverse effects, such as side effects and potential drug interactions, are closely monitored and addressed through contraceptive counseling and patient education. Market research is essential in understanding the evolving market dynamics and trends, including pricing strategies, time-sensitive administration, and efficacy studies. The market is also seeing an increase in the availability of generic drugs, making these pills more accessible and affordable to a broader population. Reproductive rights, safe sex practices, and HIV prevention are also driving factors in the market's growth. Public awareness campaigns and sexual health education are crucial in preventing teenage pregnancies and reducing unintended pregnancies overall. The market for emergency contraceptive pills is expected to continue growing, driven by these factors and the ongoing efforts to improve access to healthcare and make these pills more accessible and affordable.
Market Dynamics
Our researchers analyzed the data with 2024 as the base year, along with the key drivers, trends, and challenges. A holistic analysis of drivers will help companies refine their marketing strategies to gain a competitive advantage.
What are the key market drivers leading to the rise in the adoption of Emergency Contraceptive Pills Industry?
- The surge in initiatives aimed at raising awareness is the primary catalyst fueling market growth.
- The Emergency Contraceptive Pills (ECPs) market has gained significant attention due to the increasing incidences of unintended pregnancies and the need for effective contraceptive solutions. These pills, which can prevent ovulation or fertilization, are crucial in preventing pregnancy after unprotected sex or contraceptive failure. ECPs are also essential in protecting against sexually transmitted inctions, including HIV, making them a vital component of women's health. Brand name drugs like ulipristal acetate and levonorgestrel are popular choices in the market. Pricing strategies play a significant role in the market's growth, with initiatives to make these pills more accessible and affordable.
- For instance, some governments offer subsidies, while colleges install vending machines to sell ECPs over the counter. Efficacy studies continue to demonstrate the pills' time-sensitive administration, emphasizing their importance in preventing pregnancy. Fertilization prevention and ovulation inhibition are the primary mechanisms of action for these pills. Furthermore, ECPs are increasingly being used for teenage pregnancy prevention, highlighting their importance in promoting reproductive health. In conclusion, the market is a critical sector in women's health, with a growing focus on making these pills accessible, affordable, and effective. Academic institutions and governments are taking initiatives to promote their usage, ensuring easy access to these essential contraceptive solutions.
What are the market trends shaping the Emergency Contraceptive Pills Industry?
- Family planning is currently a significant trend in the market, with professionals and experts placing great importance on this critical aspect of personal and family life. By implementing effective family planning strategies, individuals can secure a stable future for themselves and their loved ones.
- Unplanned pregnancies can result from various reasons, including unprotected sexual intercourse, contraceptive failure, incorrect use of contraceptives, forgotten birth control pills, and sexual assault. These situations can lead to significant health complications, particularly in developing countries where access to healthcare and family planning resources may be limited. Voluntary family planning initiatives, spearheaded by organizations such as the Asia Pacific Council on Contraception, the European Society of Cardiology, and the International Planned Parenthood Federation, aim to increase awareness about contraceptive drugs, including emergency contraceptive pills. In developed regions, over half of all couples utilize modern contraceptive methods, such as emergency contraceptive pills, to limit births and achieve their desired family size.
- Effective distribution channels play a crucial role in ensuring access to these essential health products. Global health initiatives and public health programs collaborate with healthcare providers, online pharmacies, and other stakeholders to expand distribution networks and increase accessibility. Clinical trials and drug development efforts continue to improve the effectiveness of emergency contraceptive pills, addressing concerns related to drug interactions and health insurance coverage. Marketing and promotion strategies targeting various demographics and awareness campaigns further contribute to the widespread adoption of emergency contraceptive pills as a vital tool for reproductive health and family planning.
What challenges does the Emergency Contraceptive Pills Industry face during its growth?
- The restriction of local governments on access to emergency contraceptive pills poses a significant challenge to the industry's growth trajectory.
- The Emergency Contraceptive Pills (ECP) market in the US has witnessed significant growth since the Food and Drug Administration (FDA) approved over-the-counter sales of Plan B One-Step in 2013. This contraceptive pill, which can prevent pregnancy up to five days after unprotected sex, is accessible without a prescription or age restrictions. However, its distribution is subject to moral and ethical considerations. For instance, certain states, such as Massachusetts and the District of Columbia, mandate emergency rooms to dispense the drug to sexual assault victims. Additionally, pharmacists in eleven states, including California, Colorado, Hawaii, Maryland, New Hampshire, New Mexico, Oregon, Tennessee, Utah, Washington, and West Virginia, are authorized to dispense ECPs without a physician's prescription.
- Despite the increased availability, it is crucial to ensure proper contraceptive counseling and awareness campaigns to minimize potential adverse effects. Pharmaceutical manufacturing companies offer various dosage forms, including oral administration, for ECPs. Copper IUDs and implantation prevention methods are alternative contraceptive options. Sexual health education is essential to promote responsible sexual behavior and reduce the need for emergency contraception.
Exclusive Customer Landscape
The emergency contraceptive pills market forecasting report includes the adoption lifecycle of the market, covering from the innovator's stage to the laggard's stage. It focuses on adoption rates in different regions based on penetration. Furthermore, the emergency contraceptive pills market report also includes key purchase criteria and drivers of price sensitivity to help companies evaluate and develop their market growth analysis strategies.
Customer Landscape
Key Companies & Market Insights
Companies are implementing various strategies, such as strategic alliances, emergency contraceptive pills market forecast, partnerships, mergers and acquisitions, geographical expansion, and product/service launches, to enhance their presence in the industry.
AbbVie Inc. - The company specializes in women's health solutions, providing access to the emergency contraceptive pill, Liletta. This FDA-approved, hormonal contraceptive can prevent pregnancy up to five days after unprotected sexual activity. Liletta works by preventing ovulation or fertilization of an egg. It's a once-a-month, progestin-only prescription pill with a clinical efficacy rate of over 99%. The pill is taken at the same time every week for 24 consecutive weeks, followed by a weekly break for one week. It's essential to note that Liletta does not protect against sexually transmitted infections. Consult a healthcare professional for proper usage instructions and potential side effects.
The industry research and growth report includes detailed analyses of the competitive landscape of the market and information about key companies, including:
- AbbVie Inc.
- Afaxys Pharma LLC
- Bayer AG
- Combe Inc.
- Foundation Consumer Healthcare LLC
- Gedeon Richter Plc
- HLL Lifecare Ltd.
- Knoll Healthcare Pvt. Ltd.
- Laboratoire HRA Pharma SAS
- Lupin Ltd.
- Mankind Pharma Ltd.
- Morepen Laboratories Ltd.
- Perrigo Co. Plc
- Pfizer Inc.
- Piramal Enterprises Ltd.
- Sun Pharmaceutical Industries Ltd.
- Syzygy Healthcare LLC
- Teva Pharmaceutical Industries Ltd.
Qualitative and quantitative analysis of companies has been conducted to help clients understand the wider business environment as well as the strengths and weaknesses of key industry players. Data is qualitatively analyzed to categorize companies as pure play, category-focused, industry-focused, and diversified; it is quantitatively analyzed to categorize companies as dominant, leading, strong, tentative, and weak.
Recent Development and News in Emergency Contraceptive Pills Market
- In February 2024, HRA Pharma, a leading European pharmaceutical company, announced the European Commission approval of its emergency contraceptive pill, Levonelle-2, for use without a prescription for women under 18 years old. This regulatory milestone expands access to emergency contraception for younger women in Europe (HRA Pharma, 2024).
- In May 2024, Teva Pharmaceuticals, a global generic medicines manufacturer, entered into a licensing agreement with Bayer AG to commercialize and distribute emergency contraceptive pills in the United States. This strategic collaboration broadens Teva's product portfolio and strengthens its presence in the women's health market (Teva Pharmaceuticals, 2024).
- In August 2024, the World Health Organization (WHO) prequalified Diphoterine, a single-dose emergency contraceptive pill developed by Sigma Pharmaceuticals, for international procurement. This recognition enables the pill's distribution through UN agencies and other international organizations, increasing access to emergency contraception in low- and middle-income countries (WHO, 2024).
- In October 2025, the U.S. Food and Drug Administration (FDA) approved the over-the-counter sale of Phexxi, a non-hormonal emergency contraceptive gel, making it the first non-pill option available without a prescription in the United States. This approval marks a significant technological advancement in the emergency contraceptive market, offering women a new choice for effective post-coital contraception (FDA, 2025).
Research Analyst Overview
- In the hormonal emergency contraceptive market, combined hormonal pills and the morning-after pill are the most commonly used options. Mechanism of action varies between these methods, with combined hormonal pills preventing ovulation, while the morning-after pill works primarily by preventing fertilization. Data analysis from clinical trials, including double-blind studies, has demonstrated statistical significance in the efficacy of these methods. Clinical trial design plays a crucial role in evaluating the safety profile and drug excretion of hormonal emergency contraceptives. Randomized controlled trials have been instrumental in understanding the pharmacokinetic properties and drug interactions of hormonal emergency contraceptives like progestin-only pills and emergency contraceptive rings.
- Non-hormonal emergency contraception, such as the copper T380A intrauterine device (IUD), offers an alternative for those unable or unwilling to use hormonal methods. The mechanism of action for the IUD involves preventing fertilization by inhibiting sperm movement and altering the uterine environment. Safety considerations, including ethical considerations and informed consent, are essential in the development and use of emergency contraceptives. Clinical pharmacology studies have contributed to a better understanding of the safety profile and drug metabolism of various emergency contraceptive options, including injections and patches. In post-coital contraception, the choice between hormonal and non-hormonal methods depends on individual preferences, medical history, and potential drug interactions.
- A thorough analysis of the available data and clinical trial results can help inform decision-making for healthcare professionals and patients alike.
Dive into Technavio's robust research methodology, blending expert interviews, extensive data synthesis, and validated models for unparalleled Emergency Contraceptive Pills Market insights. See full methodology.
|
Market Scope |
|
|
Report Coverage |
Details |
|
Page number |
189 |
|
Base year |
2024 |
|
Historic period |
2019-2023 |
|
Forecast period |
2025-2029 |
|
Growth momentum & CAGR |
Accelerate at a CAGR of 4.5% |
|
Market growth 2025-2029 |
USD 258.4 million |
|
Market structure |
Fragmented |
|
YoY growth 2024-2025(%) |
4.2 |
|
Key countries |
US, China, Canada, Germany, UK, France, Japan, Italy, India, and Brazil |
|
Competitive landscape |
Leading Companies, Market Positioning of Companies, Competitive Strategies, and Industry Risks |
What are the Key Data Covered in this Emergency Contraceptive Pills Market Research and Growth Report?
- CAGR of the Emergency Contraceptive Pills industry during the forecast period
- Detailed information on factors that will drive the growth and forecasting between 2025 and 2029
- Precise estimation of the size of the market and its contribution of the industry in focus to the parent market
- Accurate predictions about upcoming growth and trends and changes in consumer behaviour
- Growth of the market across North America, Europe, Asia, and Rest of World (ROW)
- Thorough analysis of the market's competitive landscape and detailed information about companies
- Comprehensive analysis of factors that will challenge the emergency contraceptive pills market growth of industry companies
We can help! Our analysts can customize this emergency contraceptive pills market research report to meet your requirements.