Human Platelet Lysate Market Size 2026-2030
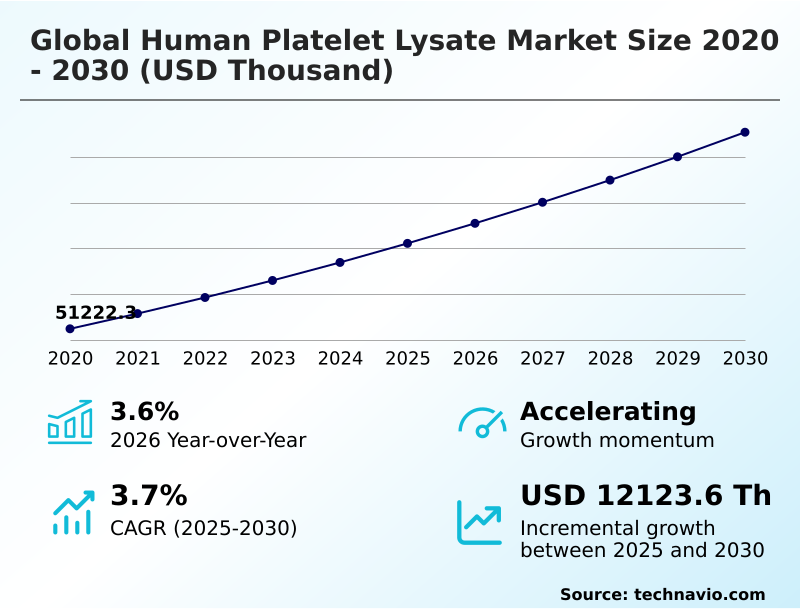
The human platelet lysate market size is valued to increase by USD 12.12 million, at a CAGR of 3.7% from 2025 to 2030. Rising prevalence of chronic diseases will drive the human platelet lysate market.
Major Market Trends & Insights
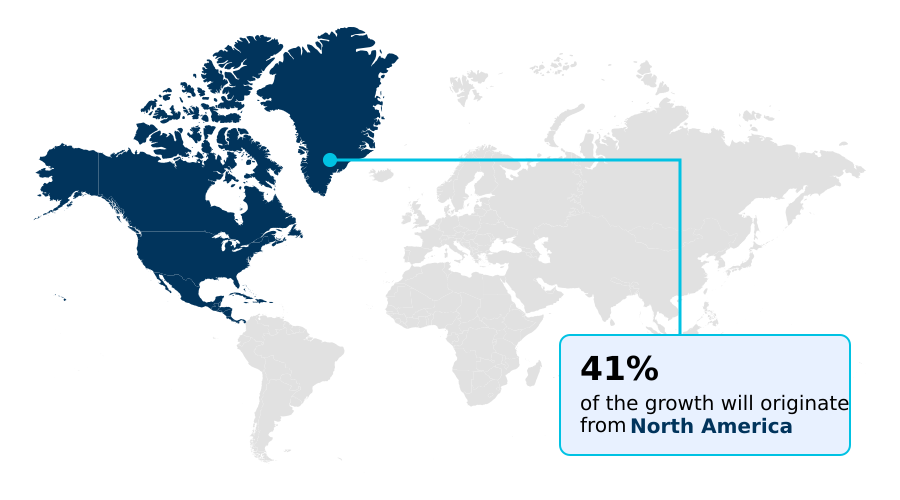
- North America dominated the market and accounted for a 41.4% growth during the forecast period.
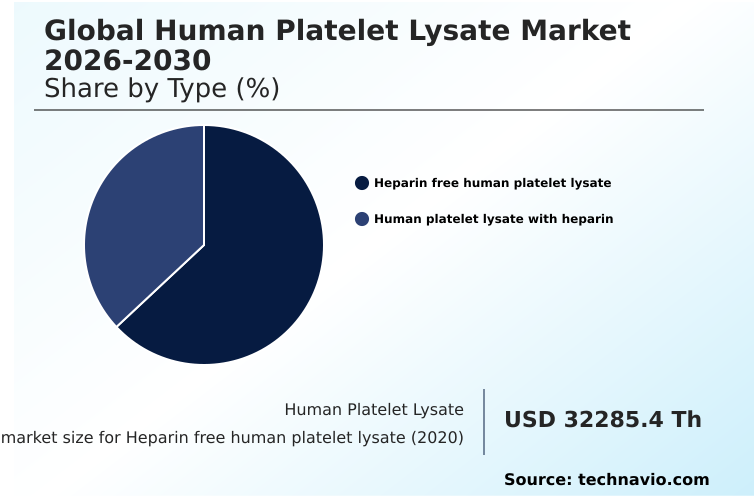
- By Type - Heparin free human platelet lysate segment was valued at USD 37.08 million in 2024
- By Application - Research use segment accounted for the largest market revenue share in 2024
Market Size & Forecast
- Market Opportunities:
- Market Future Opportunities: USD 12.12 million
- CAGR from 2025 to 2030 : 3.7%
Market Summary
- The human platelet lysate market is advancing as a critical enabler for regenerative medicine and cell therapy. Driven by a pronounced shift away from animal-derived serums, the demand for high-quality, xeno-free alternatives is accelerating. This is particularly evident in the development of treatments for chronic and degenerative diseases, where reproducible and safe cell expansion is paramount.
- A key trend is the innovation in product formulations, including lyophilized and pathogen-reduced versions that offer enhanced stability and safety, addressing logistical and regulatory concerns. However, the market faces challenges related to the standardization of manufacturing processes and the inherent variability of donor-derived materials.
- For instance, a mid-sized biotech firm preparing for clinical trials must weigh the benefits of a highly consistent, albeit more expensive, GMP-grade product against a research-grade equivalent to manage its development budget without compromising its regulatory submission. This scenario highlights the strategic balancing act companies must perform between cost, quality, and compliance.
- The increasing investment in personalized medicine and allogeneic therapies further underscores the need for scalable and reliable ancillary materials, positioning human platelet lysate as a cornerstone of next-generation biomanufacturing.
What will be the Size of the Human Platelet Lysate Market during the forecast period?
Get Key Insights on Market Forecast (PDF) Request Free Sample
How is the Human Platelet Lysate Market Segmented?
The human platelet lysate industry research report provides comprehensive data (region-wise segment analysis), with forecasts and estimates in "USD thousand" for the period 2026-2030, as well as historical data from 2020-2024 for the following segments.
- Type
- Heparin free human platelet lysate
- Human platelet lysate with heparin
- Application
- Research use
- Clinical use
- Product type
- Liquid HPL
- Lyophilized HPL
- Customized HPL
- Geography
- North America
- US
- Canada
- Mexico
- Europe
- Germany
- France
- UK
- Asia
- Rest of World (ROW)
- North America
By Type Insights
The heparin free human platelet lysate segment is estimated to witness significant growth during the forecast period.
The human platelet lysate market is increasingly defined by the adoption of heparin-free formulations, which now account for over 60% of the market. This shift is driven by the needs of advanced therapy medicinal products and biopharmaceutical manufacturing.
For cell-based therapies, avoiding heparin is critical as it can interfere with cellular processes, a key consideration in both clinical research and GMP-grade production.
As the industry moves away from traditional human platelet lysate vs FBS comparisons, the focus on optimized, high-purity human-derived products intensifies.
This segment's growth underscores the demand for superior cell therapy raw materials that support robust HPL for MSC expansion and the development of therapeutic cell preparations, aligning with stringent standards for GMP manufacturing of cell therapies and cell culture media optimization.
The Heparin free human platelet lysate segment was valued at USD 37.08 million in 2024 and showed a gradual increase during the forecast period.
Regional Analysis
North America is estimated to contribute 41.4% to the growth of the global market during the forecast period.Technavio’s analysts have elaborately explained the regional trends and drivers that shape the market during the forecast period.
See How Human Platelet Lysate Market Demand is Rising in North America Request Free Sample
The geographic landscape of the human platelet lysate market is led by North America, which is projected to contribute over 41% of the incremental growth, driven by its advanced cell therapy manufacturing infrastructure and robust funding for regenerative medicine.
This region's dominance is supported by a clear regulatory environment facilitating the use of GMP-compliant manufacturing. Meanwhile, Asia is the fastest-growing region, accounting for over 18% of market expansion, with countries rapidly scaling their biopharmaceutical capabilities.
The demand for xeno-free alternatives and fibrinogen-depleted products is a global phenomenon.
Key players are establishing a resilient cell therapy supply chain to serve the needs of commercial cell therapy manufacturing worldwide, ensuring the availability of clinical-grade material for expanding applications in advanced therapies and gene therapies, including the cultivation of mesenchymal stromal cells.
Market Dynamics
Our researchers analyzed the data with 2025 as the base year, along with the key drivers, trends, and challenges. A holistic analysis of drivers will help companies refine their marketing strategies to gain a competitive advantage.
- The evolution of the human platelet lysate market reflects a strategic journey from academic research to mainstream clinical application. Key considerations for developers include understanding heparin free human platelet lysate advantages, especially in sensitive cell culture environments where anticoagulants can interfere with outcomes.
- For therapeutic applications, the use of gamma-irradiated human platelet lysate for clinical use is becoming a standard for ensuring virological safety. The industry is also actively engaged in developing artificial human platelet lysate solutions to overcome the limitations of donor-based sourcing. This move towards synthetic biology paves the way for customized human platelet lysate for specific cell types.
- The application scope is broadening, with promising results seen in using human platelet lysate for nerve injury repair and its positive impact of human platelet lysate on neurite outgrowth. Furthermore, the role of human platelet lysate in bioprinting and human platelet lysate in dermatology and wound healing showcases its versatility.
- A critical step for labs is optimizing human platelet lysate concentration in media, as this directly affects cell proliferation. For many, transitioning from fbs to human platelet lysate is a pivotal decision driven by regulatory and ethical factors. The demand for GMP-grade human platelet lysate for commercial manufacturing is surging, particularly when using human platelet lysate for MSC therapy.
- A thorough cost-benefit analysis of lyophilized human platelet lysate often reveals long-term savings in logistics and storage, with some facilities reporting a 30% reduction in waste associated with expired liquid product. Rigorous quality control assays for human platelet lysate are non-negotiable for products intended for human platelet lysate in advanced therapy medicinal products.
- The industry is also addressing human platelet lysate supply chain and sourcing ethics. Emerging human platelet lysate applications in ophthalmology and its role in cardiac tissue repair signal future growth, while researchers continue to find solutions for overcoming gel formation in human platelet lysate.
- Navigating the regulatory pathway for human platelet lysate based therapies remains a key focus for all stakeholders.
What are the key market drivers leading to the rise in the adoption of Human Platelet Lysate Industry?
- The rising global prevalence of chronic diseases is a primary driver for the market, creating a pressing need for advanced therapeutic solutions and innovative cell-based therapies.
- The market's growth is primarily driven by the expanding field of regenerative medicine, which relies on high-quality cell culture supplements for developing autologous therapies and allogeneic cell therapies.
- The unique composition of growth factors and cytokines in human platelet lysate makes it ideal for the cultivation of mesenchymal stem cells.
- Demand is further fueled by the development of exosome-rich platelet lysate for advanced therapeutic uses, including HPL for CAR-T cell culture. Adherence to HPL quality control standards is critical, with automated systems improving compliance by over 95%.
- As organizations refine the human platelet lysate production process to support both autologous vs allogeneic HPL applications, the need for clear regulatory requirements for HPL becomes more pressing.
- The use of cryopreserved platelets as a starting material is also streamlining manufacturing workflows.
What are the market trends shaping the Human Platelet Lysate Industry?
- Growing investments in stem cell research represent a significant market trend. This is driven by the vast therapeutic potential of stem cells, which requires high-quality cell culture supplements for effective expansion.
- Key trends are reshaping the human platelet lysate market, with a strong emphasis on product innovation and safety. The development of lyophilized HPL is a significant advancement, offering a stable alternative that reduces cold-chain logistical costs by up to 40% compared to liquid HPL. This enhances the feasibility of large-scale clinical applications of HPL.
- Concurrently, the introduction of pathogen-reduced platelet lysate through methods like viral inactivation addresses critical safety concerns, making it a preferred option for clinical-grade HPL. Innovations in serum-free media are further improving batch-to-batch consistency, a crucial factor for reproducible results in a cell proliferation assay and large-scale in vitro cell expansion.
- Firms are increasingly differentiating between lyophilized vs liquid HPL for applications such as HPL in orthopedic regeneration, where consistency is paramount, driving a market shift toward more stable and reliable formats.
What challenges does the Human Platelet Lysate Industry face during its growth?
- Stringent regulations and quality control requirements for human-derived products pose a significant challenge, impacting both the development and commercialization of new solutions within the industry.
- Key challenges in the human platelet lysate market center on manufacturing complexities and regulatory hurdles. The cell therapy bioprocessing of this ancillary material requires significant investment to ensure a consistent proteomic profile and preserve its immunomodulatory properties. Establishing a robust cell culture system for xeno-free cell culture presents difficulties, particularly in scaling up production for clinical trial materials.
- For instance, creating a functional tissue engineering scaffold with embedded cells relies on the lysate's quality, where inconsistencies can lead to a 20% failure rate in tissue construct development. The management of extracellular vesicles within the lysate adds another layer of complexity to bioprocessing.
- As the industry pushes for therapeutic advancements in cell therapy and stem cell treatments, overcoming these technical and supply chain challenges is paramount for market progress.
Exclusive Technavio Analysis on Customer Landscape
The human platelet lysate market forecasting report includes the adoption lifecycle of the market, covering from the innovator’s stage to the laggard’s stage. It focuses on adoption rates in different regions based on penetration. Furthermore, the human platelet lysate market report also includes key purchase criteria and drivers of price sensitivity to help companies evaluate and develop their market growth analysis strategies.
Customer Landscape of Human Platelet Lysate Industry
Competitive Landscape
Companies are implementing various strategies, such as strategic alliances, human platelet lysate market forecast, partnerships, mergers and acquisitions, geographical expansion, and product/service launches, to enhance their presence in the industry.
Amerigo Scientific - Specializes in developing advanced human-derived products, including GMP-grade human platelet lysate supplements, for cell culture and tissue regeneration applications.
The industry research and growth report includes detailed analyses of the competitive landscape of the market and information about key companies, including:
- Amerigo Scientific
- AventaCell BioMedical Corp
- BBI Solutions OEM Ltd.
- BioLife Solutions Inc.
- Captivate Bio
- Compass Biomedical Inc.
- Creative Biolabs
- GeminiBio
- Lonza Group Ltd.
- Macopharma SA
- Merck KGaA
- Mill Creek Life Sciences Inc.
- MP Biomedicals Inc.
- PAN-Biotech GmbH
- PL BioScience GmbH
- Sartorius CellGenix GmbH
- Serana Europe GmbH
- STEMCELL Technologies Inc.
- TRINOVA BIOCHEM GmbH
- ZenBio Inc.
Qualitative and quantitative analysis of companies has been conducted to help clients understand the wider business environment as well as the strengths and weaknesses of key industry players. Data is qualitatively analyzed to categorize companies as pure play, category-focused, industry-focused, and diversified; it is quantitatively analyzed to categorize companies as dominant, leading, strong, tentative, and weak.
Recent Development and News in Human platelet lysate market
- In August 2024, PL BioScience GmbH secured substantial Series A funding to expand production capabilities for its human platelet lysate products.
- In April 2025, PL BioScience GmbH introduced a gamma-irradiated, Good Manufacturing Practice (GMP) grade human platelet lysate, enhancing the virological safety of the cell culture supplement for clinical use.
- In May 2025, PL BioScience GmbH announced a partnership with DewCell Biotherapeutics to produce the world's first artificial human platelet lysate solution from lab-grown platelets, addressing supply chain constraints.
- In August 2025, Captivate Bio announced that PLUS Human Platelet Lysate products from Compass Biomedical Inc. would be available through its Access Program, widening distribution of GMP-grade HPL for large-scale research.
Dive into Technavio’s robust research methodology, blending expert interviews, extensive data synthesis, and validated models for unparalleled Human Platelet Lysate Market insights. See full methodology.
| Market Scope | |
|---|---|
| Page number | 280 |
| Base year | 2025 |
| Historic period | 2020-2024 |
| Forecast period | 2026-2030 |
| Growth momentum & CAGR | Accelerate at a CAGR of 3.7% |
| Market growth 2026-2030 | USD 12123.6 thousand |
| Market structure | Fragmented |
| YoY growth 2025-2026(%) | 3.6% |
| Key countries | US, Canada, Mexico, Germany, France, UK, Italy, Spain, The Netherlands, China, Japan, India, South Korea, Indonesia, Thailand, Brazil, Saudi Arabia, South Africa, UAE, Israel, Turkey, Argentina and Colombia |
| Competitive landscape | Leading Companies, Market Positioning of Companies, Competitive Strategies, and Industry Risks |
Research Analyst Overview
- The human platelet lysate market is fundamentally reshaping the landscape of cell-based therapies by providing a superior xeno-free alternative to traditional supplements. Its integral role as a cell culture supplement, rich in growth factors and cytokines, is critical for the in vitro cell expansion of mesenchymal stem cells and other cells used in regenerative medicine.
- The industry is advancing toward GMP-grade and clinical-grade HPL, with a focus on developing specialized heparin-free formulations and lyophilized HPL to improve batch-to-batch consistency and logistical efficiency. Innovations in viral inactivation and the development of allogeneic cell therapies are expanding the applications of autologous therapies. Within cell therapy bioprocessing, the proteomic profile and immunomodulatory properties of the lysate are optimized.
- A key boardroom consideration is the adoption of advanced ancillary material, which can accelerate development timelines; for instance, using a premium clinical-grade HPL has been shown to reduce cell proliferation assay validation time by up to 25%. This shift is vital for biopharmaceutical manufacturing of therapeutic cell preparations.
- As the use of serum-free media becomes standard in xeno-free cell culture, human platelet lysate, including cryopreserved platelets and fibrinogen-depleted variants, is established as a key component in GMP-compliant manufacturing for advanced therapy medicinal products and tissue engineering scaffolds, with its extracellular vesicles gaining research interest.
- The expansion of mesenchymal stromal cells using platelet-derived growth factors is a cornerstone of many clinical research programs.
What are the Key Data Covered in this Human Platelet Lysate Market Research and Growth Report?
-
What is the expected growth of the Human Platelet Lysate Market between 2026 and 2030?
-
USD 12.12 million, at a CAGR of 3.7%
-
-
What segmentation does the market report cover?
-
The report is segmented by Type (Heparin free human platelet lysate, and Human platelet lysate with heparin), Application (Research use, and Clinical use), Product Type (Liquid HPL, Lyophilized HPL, and Customized HPL) and Geography (North America, Europe, Asia, Rest of World (ROW))
-
-
Which regions are analyzed in the report?
-
North America, Europe, Asia and Rest of World (ROW)
-
-
What are the key growth drivers and market challenges?
-
Rising prevalence of chronic diseases, Stringent regulations and quality control requirements for human-derived products
-
-
Who are the major players in the Human Platelet Lysate Market?
-
Amerigo Scientific, AventaCell BioMedical Corp, BBI Solutions OEM Ltd., BioLife Solutions Inc., Captivate Bio, Compass Biomedical Inc., Creative Biolabs, GeminiBio, Lonza Group Ltd., Macopharma SA, Merck KGaA, Mill Creek Life Sciences Inc., MP Biomedicals Inc., PAN-Biotech GmbH, PL BioScience GmbH, Sartorius CellGenix GmbH, Serana Europe GmbH, STEMCELL Technologies Inc., TRINOVA BIOCHEM GmbH and ZenBio Inc.
-
Market Research Insights
- The human platelet lysate market is shaped by a dynamic interplay of scientific innovation and clinical demand. The growing preference for serum-free cell culture media is a significant factor, with adoption rates in new clinical projects increasing by over 20% in recent years.
- This shift supports the expansion of clinical-grade cell therapies, which now underpin more than 120 active clinical trials globally. As GMP manufacturing of cell therapies matures, the focus on the cell therapy supply chain intensifies, demanding robust sourcing and quality control.
- Organizations are leveraging advanced therapies to address complex diseases, a strategy that improves therapeutic outcomes by an estimated 15% compared to conventional treatments. This progress in gene therapies and other advanced modalities reinforces the critical role of high-quality human platelet lysate in enabling next-generation medical breakthroughs.
We can help! Our analysts can customize this human platelet lysate market research report to meet your requirements.