Renal Anemia Therapeutics Market Size 2025-2029
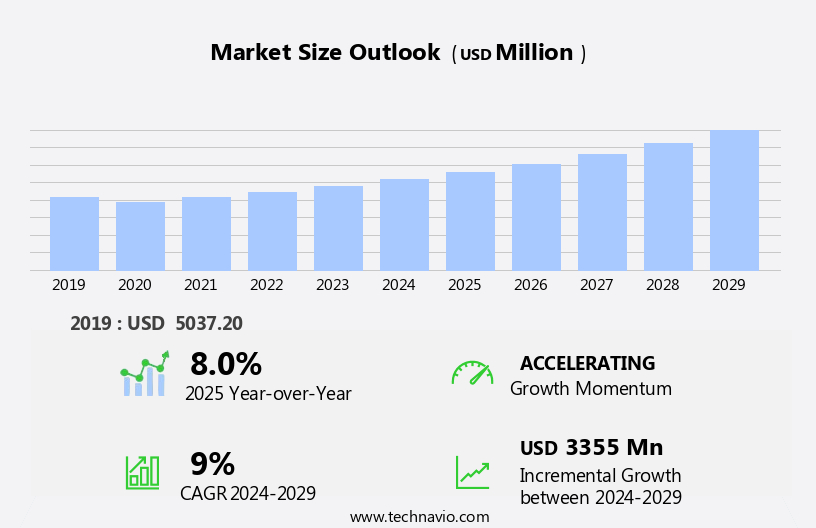
The renal anemia therapeutics market size is forecast to increase by USD 3.36 billion, at a CAGR of 9% between 2024 and 2029.
- The market is driven by the substantial patient population suffering from Chronic Kidney Disease (CKD), making it a significant market with immense growth potential. This large patient base necessitates continuous development of effective treatments for managing renal anemia. Pharmacodynamic profiles are being optimized to minimize adverse events, while drug delivery systems are being developed to enhance patient compliance through convenient dosage regimens and administration methods. Another key trend in the market is the increasing adoption of biosimilars, which offer cost-effective alternatives to branded therapies, thereby expanding market access and affordability. However, the side-effects associated with the oral administration of renal anemia drugs pose a significant challenge to market growth.
- These side-effects, including gastrointestinal disturbances and potential cardiovascular risks, necessitate the exploration of alternative delivery methods and the development of safer, more effective treatments. Companies seeking to capitalize on market opportunities should focus on addressing these challenges through innovative product development and strategic partnerships, ensuring they stay competitive in this dynamic market landscape. Genetic testing plays a crucial role in personalized medicine, allowing for precision dosing and improved patient outcomes.
What will be the Size of the Renal Anemia Therapeutics Market during the forecast period?
Explore in-depth regional segment analysis with market size data - historical 2019-2023 and forecasts 2025-2029 - in the full report.
Request Free Sample
- The market is experiencing significant activity and trends, driven by the biopharmaceutical industry's continuous efforts to develop effective treatment options for this debilitating condition. Licensing agreements have been a prominent feature in the market, enabling collaborations between companies and research institutions to advance innovation. Marketing and sales strategies are evolving to address health disparities and improve healthcare utilization for patients with renal anemia. Future trends include a focus on patient outcomes, intellectual property protection, and health economics. Anemia management remains a priority in long-term care settings, with patient advocacy groups pushing for greater health equity and disease progression monitoring.
- Public health initiatives aim to reduce healthcare costs by improving access to affordable treatments and optimizing care delivery. The global health community recognizes the importance of addressing renal anemia to improve patient quality of life and overall healthcare outcomes. The market is also driven by the increasing adoption of biosimilars, which offer cost-effective alternatives to branded drugs.
How is this Renal Anemia Therapeutics Industry segmented?
The renal anemia therapeutics industry research report provides comprehensive data (region-wise segment analysis), with forecasts and estimates in "USD million" for the period 2025-2029, as well as historical data from 2019-2023 for the following segments.
- Type
- IV
- Oral
- Distribution Channel
- Hospital pharmacy
- Retail pharmacy
- Online pharmacy
- Form Factor
- Solid
- Liquid
- Geography
- North America
- US
- Canada
- Europe
- France
- Germany
- Italy
- UK
- APAC
- China
- India
- Japan
- South America
- Brazil
- Rest of World (ROW)
- North America
By Type Insights
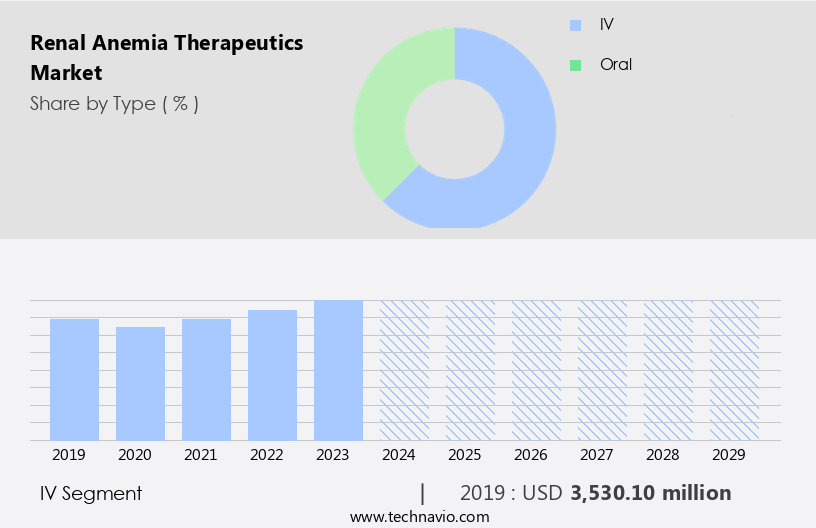
The IV segment is estimated to witness significant growth during the forecast period. The market is witnessing significant growth, driven by the increasing prevalence of chronic kidney disease and the need for effective treatments to manage associated anemia. Overall, the market is expected to experience steady growth In the coming years, driven by the increasing prevalence of CKD and the availability of cost-effective treatment options. Erythropoiesis-stimulating agents (ESAs) remain a cornerstone of therapy, improving hemoglobin levels and enhancing quality of life for patients. However, challenges such as drug resistance, drug interactions, and adverse events necessitate ongoing research and development.
Phase III trials are underway for novel therapies, including gene therapy, stem cell therapy, and targeted drug delivery. Animal models are used extensively in preclinical studies to evaluate drug efficacy, metabolism, and safety. Regulatory approvals, such as EMA approval, are essential for market access and reimbursement policies. Drug formulation and stability are critical considerations for drug development, with intravenous (IV) administration remaining a popular choice due to its high therapeutic index and rapid onset of action. Iron chelators are used to manage iron overload, a common complication of long-term ESAs use. Drug safety and patient education are essential to ensure proper use and minimize adverse events.
Intravenous iron, such as ferric carboxymaltose, is gaining popularity due to its controlled delivery to the mononuclear phagocytic system, reducing the risk of ionic iron release. Drug metabolism and excretion are key factors in drug development, with pharmacokinetic profiles and drug interactions under close scrutiny. Peritoneal dialysis and disease management strategies are also being explored to optimize treatment outcomes for patients with end-stage renal disease. Target identification and drug discovery are ongoing efforts to develop novel therapies and expand the therapeutic landscape for renal anemia. The market caters to the unmet medical needs of patients suffering from chronic kidney disease (CKD), a leading cause of chronic disease worldwide.
The IV segment was valued at USD 3.53 billion in 2019 and showed a gradual increase during the forecast period.
The Renal Anemia Therapeutics Market is witnessing rapid advancements, driven by innovations in the biopharmaceutical industry. With a growing patient population affected by chronic kidney disease, companies are developing cutting-edge treatments to address anemia-related complications. Effective business strategies play a vital role in enhancing drug development, regulatory approvals, and market expansion, ensuring wider availability of advanced therapies. A key focus remains on improving access to healthcare, making novel therapeutics more affordable and accessible to patients globally. Anemia in CKD patients increases the risk of mortality and contributes to cardiovascular complications, such as heart failure, stroke, and myocardial infarction.
Regional Analysis
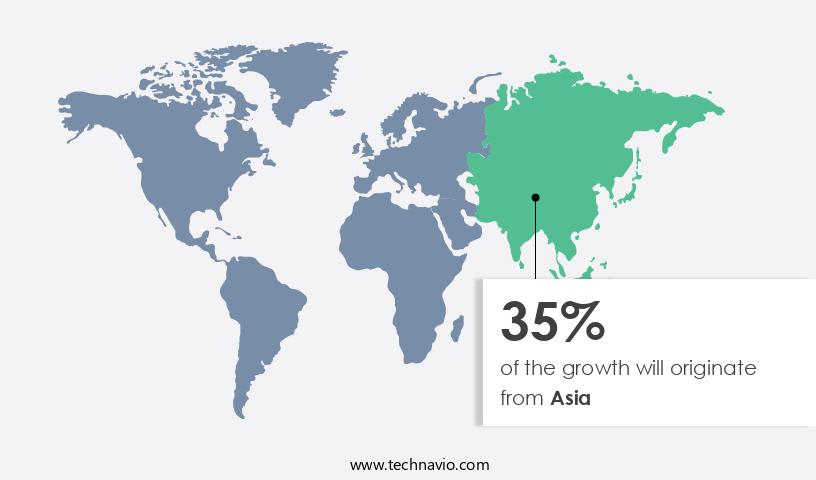
Asia is estimated to contribute 35% to the growth of the global market during the forecast period. Technavio's analysts have elaborately explained the regional trends and drivers that shape the market during the forecast period.
The market in North America is significantly larger than in other regions due to advanced healthcare systems, providing high-quality treatment for anemia, primarily through clinics and hospitals. The US and Canada's increasing healthcare expenditure fuel market growth. Factors such as substantial investment in the treatment of Chronic Kidney Disease (CKD) and associated ailments, including renal anemia, and the presence of several key players, make the US a significant contributor to the market's revenue in the Americas. Erythropoiesis-stimulating agents, iron chelators, and stem cell therapies dominate the market, with a focus on improving quality of life, drug delivery, dosage regimens, and patient compliance.
Phase III trials, physician education, and patient education are crucial for regulatory approvals, ensuring drug safety and efficacy. Precision medicine and genetic engineering are emerging trends, with gene therapy and targeted drug delivery showing promise. Drug development involves extensive preclinical studies, pharmacodynamic and pharmacokinetic profiles, and drug metabolism. Drug formulation, therapeutic index, and drug interactions are essential considerations. Intravenous and subcutaneous administrations, intravenous iron, and oral iron supplements are common drug delivery methods. Market access, reimbursement policies, and health insurance coverage are significant challenges, with end-stage renal disease patients requiring long-term treatment. Peritoneal dialysis and disease management are crucial aspects of treatment guidelines.
Regulatory approvals, such as EMA approval, play a vital role in market access. Drug stability, drug excretion, and drug resistance are essential factors in drug development and market success. Market dynamics and trends evolve as new therapeutic approaches, such as stem cell therapy and gene therapy, emerge, offering potential solutions for renal anemia patients. The development of novel therapeutic agents and drug delivery systems, as well as the expansion of healthcare facilities, are expected to provide significant opportunities for market participants.
Market Dynamics
Our researchers analyzed the data with 2024 as the base year, along with the key drivers, trends, and challenges. A holistic analysis of drivers will help companies refine their marketing strategies to gain a competitive advantage.
What are the Renal Anemia Therapeutics market drivers leading to the rise in the adoption of Industry?
- The substantial population of chronic kidney disease (CKD) patients serves as the primary market driver. The increasing prevalence of chronic kidney disease (CKD) is driving the demand for effective renal anemia therapeutics. Patients with conditions such as diabetes and hypertension are at a higher risk of developing CKD, which can lead to anemia due to the impaired production of erythropoietin (EPO) in the kidneys. EPO is a hormone that stimulates the production of red blood cells (RBCs). In the absence of sufficient EPO, the body is unable to produce the required number of RBCs, leading to anemia. Several therapeutic approaches are being explored to address renal anemia, including lead optimization of erythropoiesis-stimulating agents (ESAs), gene therapy, and pharmacodynamic profile enhancement.
- Genetic testing is also being utilized to identify the underlying causes of anemia and tailor treatment regimens accordingly. Phase III trials are ongoing to evaluate the safety and efficacy of novel therapeutic agents. Drug delivery systems and dosage regimens are being optimized to improve patient compliance and enhance the therapeutic response. Patient education and physician education are crucial components of the treatment strategy to ensure optimal outcomes. Animal models are being used to study the mechanisms of renal anemia and to evaluate the efficacy of potential therapeutics.
What are the Renal Anemia Therapeutics market trends shaping the Industry?
- The increasing utilization of biosimilars represents a significant market trend in the healthcare industry. Biosimilars, which are biologically similar to original innovative biologic medicines, are gaining acceptance due to their cost-effectiveness and similar clinical efficacy. Biosimilar medications, which functionally replicate their reference biologics in terms of structure, efficacy, and safety, have gained significance in the healthcare industry due to their potential cost savings. In the context of renal anemia therapeutics, biosimilar epoetins have been a subject of interest. These agents have been accessible in Europe for a decade and have proven beneficial for patients with chronic renal disease. The regulatory approval process for biosimilars is stringent, ensuring they meet the same safety, effectiveness, and quality standards as their reference medications. The use of biosimilar epoetins in treating renal anemia results in significant cost savings without compromising patient care.
- Drug distribution, absorption, metabolism, and safety are crucial factors in the development and administration of these medications. Preclinical studies and targeted drug delivery methods, such as intravenous administration of intravenous iron, are being explored to optimize treatment outcomes. Regulatory approvals for these therapies are underway, with an emphasis on precision medicine and drug safety. Intravenous iron, as a component of these therapies, plays a vital role in addressing the iron deficiency often associated with chronic renal disease. Iron chelators are used to minimize the side effects of iron overload. The market dynamics for renal anemia therapeutics are influenced by these factors, along with treatment guidelines and ongoing research.
How does Renal Anemia Therapeutics market face challenges during its growth?
- The oral administration of renal anemia drugs poses significant side-effects, which represent a major challenge and hinder the growth of the industry. Anemia, a common complication in chronic kidney disease (CKD) patients, can significantly impact their quality of life. Conventional oral therapies, such as oral iron supplements, have limitations due to poor gastrointestinal (GI) tolerance and absorption. These challenges have led to the exploration of alternative treatment options. The market is witnessing significant growth due to the development of innovative therapies. EMA approval of subcutaneous administration of erythropoiesis-stimulating agents (ESAs) and intravenous iron supplements has revolutionized the treatment landscape. These new therapies offer better efficacy, fewer side effects, and improved patient compliance. Clinical trials and clinical research are underway to evaluate the safety and efficacy of these new therapies.
- Adverse events, such as those related to GI tolerance, are being closely monitored. Drug discovery efforts are focused on improving drug stability and addressing the challenges associated with reimbursement policies. The limitations of oral iron drugs, including poor absorption and side effects, are driving the demand for alternative therapies. Inflammation due to CKD increases the production of hepcidin, a protein that controls iron homeostasis, further limiting the absorption of oral iron drugs. The market dynamics are shaped by these factors and the ongoing clinical research in the field.
Exclusive Customer Landscape
The renal anemia therapeutics market forecasting report includes the adoption lifecycle of the market, covering from the innovator's stage to the laggard's stage. It focuses on adoption rates in different regions based on penetration. Furthermore, the renal anemia therapeutics market report also includes key purchase criteria and drivers of price sensitivity to help companies evaluate and develop their market growth analysis strategies.
Customer Landscape
Key Companies & Market Insights
Companies are implementing various strategies, such as strategic alliances, renal anemia therapeutics market forecast, partnerships, mergers and acquisitions, geographical expansion, and product/service launches, to enhance their presence in the industry.
Akebia Therapeutics - The company specializes in renal anemia therapeutics, including the development of innovative treatments for renal anemia.
The industry research and growth report includes detailed analyses of the competitive landscape of the market and information about key companies, including:
- Akebia Therapeutics
- Amgen Inc.
- Astellas Pharma Inc.
- AstraZeneca Plc
- Bayer AG
- CSL Ltd.
- Daiichi Sankyo Co. Ltd.
- Dr Reddys Laboratories Ltd.
- F. Hoffmann La Roche Ltd.
- FibroGen Inc.
- GlaxoSmithKline Plc
- Japan Tobacco Inc.
- JCR Pharmaceticals Co. Ltd.
- Kirin Holdings Co. Ltd.
- Mitsubishi Chemical Group Corp.
- Pfizer Inc.
- Pharmacosmos AS
- Sun Pharmaceutical Industries Ltd.
- Travere Therapeutics Inc.
Qualitative and quantitative analysis of companies has been conducted to help clients understand the wider business environment as well as the strengths and weaknesses of key industry players. Data is qualitatively analyzed to categorize companies as pure play, category-focused, industry-focused, and diversified; it is quantitatively analyzed to categorize companies as dominant, leading, strong, tentative, and weak.
Recent Development and News in Renal Anemia Therapeutics Market
- In March 2024, Amgen Inc. announced the U.S. Food and Drug Administration (FDA) approval of its Epoetin alfa biosimilar, Movencite-F, for the treatment of anemia in chronic kidney disease (CKD) patients. This approval marks the entry of Amgen's biosimilar into the market, intensifying competition among market leaders (Amgen Press Release, 2024).
- In August 2024, Fresenius Medical Care and AstraZeneca entered into a strategic collaboration to develop and commercialize biosimilar erythropoietin and peginesatide in dialysis facilities and outpatient clinics. This partnership aims to expand the offerings of both companies in the market (Fresenius Medical Care Press Release, 2024).
- In January 2025, the FDA granted priority review to Adolor Corporation's new drug application (NDA) for Zegalipro, an investigational, once-weekly subcutaneous injection for the treatment of anemia in CKD patients. This development signifies a potential technological advancement in the market, offering patients a more convenient treatment option (Adolor Corporation Press Release, 2025).
- In March 2025, Janssen Pharmaceuticals and Merck KGaA's collaboration on the development and commercialization of luspatercept for the treatment of anemia in patients with beta-thalassemia and myelodysplastic syndromes (MDS) received a positive opinion from the European Medicines Agency's Committee for Medicinal Products for Human Use (CHMP). This approval is expected to expand the reach of this treatment in Europe and further solidify the market position of Janssen and Merck KGaA (Janssen Pharmaceuticals Press Release, 2025).
Research Analyst Overview
The market continues to evolve as researchers and pharmaceutical companies explore new approaches to address the complexities of treating anemia in chronic kidney disease (CKD) patients. Erythropoiesis-stimulating agents (ESAs), which have been a mainstay of treatment for decades, are being supplemented by innovative therapies such as gene therapy, stem cell therapy, and iron chelators. ESA approval by regulatory bodies like the European Medicines Agency (EMA) for subcutaneous administration has expanded treatment options and improved patient quality of life. Hemoglobin level targets and dosage regimens are under constant review to optimize treatment outcomes and minimize adverse events.
Health insurance coverage and reimbursement policies remain critical factors in market dynamics, influencing clinical trial design and patient access to new therapies. Ongoing clinical research in areas like precision medicine, drug discovery, and drug development seeks to improve drug stability, efficacy, and therapeutic index. Adverse events, such as iron overload from oral iron supplements, have spurred innovation in drug delivery systems, including targeted drug delivery and intravenous administration. Drug safety and distribution are also key concerns, with ongoing efforts to improve patient education and physician education. Market access and regulatory approvals for new therapies, such as intravenous iron and erythropoiesis-stimulating agents, continue to shape the competitive landscape.
Market trends toward personalized medicine and disease management are driving the need for advanced drug formulations and treatment guidelines. Preclinical studies and animal models provide valuable insights into drug metabolism, pharmacodynamic profile, and drug resistance. Pharmacokinetic and pharmacodynamic profiling are essential for optimizing drug dosing and minimizing drug interactions. The evolving nature of renal anemia therapeutics necessitates a dynamic approach to market analysis, with ongoing research and development efforts in areas like drug discovery, drug stability, and regulatory approvals shaping the future of the market.
Dive into Technavio's strong research methodology, blending expert interviews, extensive data synthesis, and validated models for unparalleled Renal Anemia Therapeutics Market insights. See full methodology.
|
Market Scope |
|
|
Report Coverage |
Details |
|
Page number |
210 |
|
Base year |
2024 |
|
Historic period |
2019-2023 |
|
Forecast period |
2025-2029 |
|
Growth momentum & CAGR |
Accelerate at a CAGR of 9% |
|
Market growth 2025-2029 |
USD 3.36 billion |
|
Market structure |
Fragmented |
|
YoY growth 2024-2025(%) |
8.0 |
|
Key countries |
US, Germany, China, Canada, UK, France, Italy, Japan, India, and Brazil |
|
Competitive landscape |
Leading Companies, Market Positioning of Companies, Competitive Strategies, and Industry Risks |
What are the Key Data Covered in this Renal Anemia Therapeutics Market Research and Growth Report?
- CAGR of the Renal Anemia Therapeutics industry during the forecast period
- Detailed information on factors that will drive the growth and forecasting between 2025 and 2029
- Precise estimation of the size of the market and its contribution of the industry in focus to the parent market
- Accurate predictions about upcoming growth and trends and changes in consumer behaviour
- Growth of the market across North America, Europe, Asia, and Rest of World (ROW)
- Thorough analysis of the market's competitive landscape and detailed information about companies
- Comprehensive analysis of factors that will challenge the renal anemia therapeutics market growth of industry companies
We can help! Our analysts can customize this renal anemia therapeutics market research report to meet your requirements.